Current projects in the Anastassov Lab focus on understanding the evolution, structure, and function of atypical retinal circuitry.
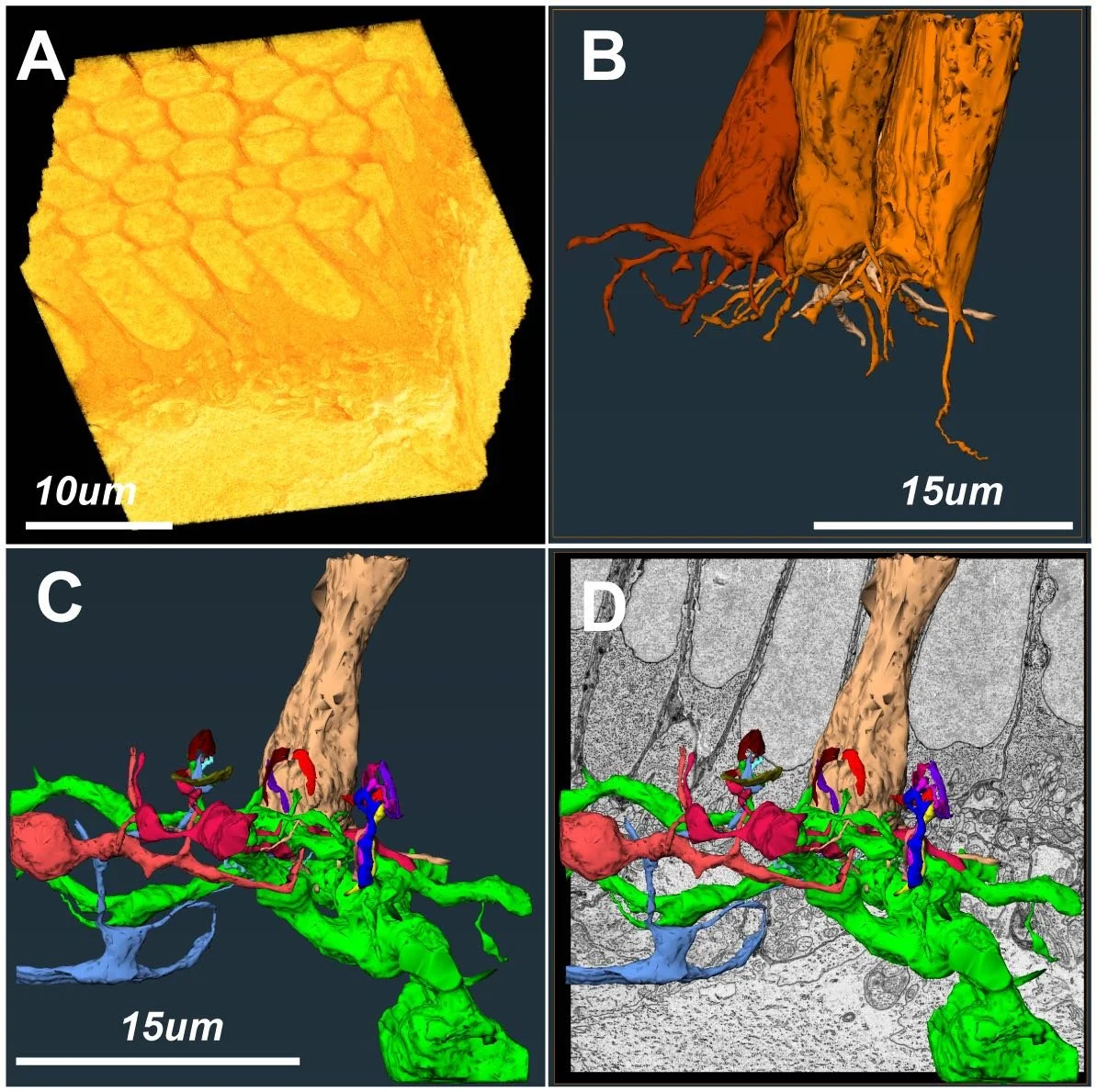
Connectomic reconstruction of the skate retina
In duplex retinae, the processing of visual information in dim and bright light conditions is clearly separated between the rod and cone system. The different physiology of rod and cone pathways is also reflected in their distinct anatomical hallmarks. Differences are starkest when one focuses on the outer segments and synaptic terminals of each cell type. In this project we are obtaining 3-dimensional reconstructions of synaptic connections between neuronal cell types in the skate retina using serial block-face 3D electron microscopy (SB-3DEM) data. This will allow us to create a map of the retinal network, known as a “connectome”. Currently, connectomic data from retinal tissue is mostly limited to the duplex (rod+cone) retinae of commonly used model organisms, like mouse, primate, or zebrafish.
Physiology of parallel circuits in a monotypic photoreceptor retina
In mixed rod-cone retinae, vision under scotopic and photopic conditions is mediated by the rod and cone circuitry, respectively. This allows for a slow, saturable detection of individual photons by rods and for a fast, un-saturable detection of multiple photons by cones. Furthermore, a key functional feature of the duplex vertebrate retina is its ability to split visual information into parallel processing channels downstream of photoreceptors, which is manifested in the anatomical and functional diversity of its neuron types and subtypes. This organization is consistently preserved across the duplex retinae of vertebrates, but our preliminary data suggests a significantly different organization in the skate retina. In this project we are investigating the physiology of the inner retina using patch clamp electrophysiology and precise spectral control of LED stimulation of the tissue.
Functional and molecular characteristics of retinal neurons in elasmobranch retina
In the dark, the pure-rod skate retina is capable of functioning at the threshold of sensitivity as a single photon detector, much like the rod-mediated scotopic vision in duplex retinae. What makes the skate retina unique, however, is its ability to function at the much brighter, i.e. photopic, levels of illumination - a range typically mediated by cone function. This light-adaptation process can be observed in the physiology of individual rod cells, as well as within the downstream retinal circuitry. Previous studies indicate that rod opsin is the only type of visual pigment detectable spectrally, or through molecular cloning in the skate retina, and very little is known about the molecular composition of cells in the downstream circuitry. These lines of evidence have thus far supported the hypothesis that rhodopsin is likely the only visual pigment mediating the functional plasticity in skate rods and have not shed any light on elements postsynaptic to rods. In this project we are investigating the functional and molecular characteristics of photoreceptors and other retinal neurons in the pure-rod skate retina using approaches like live cell Ca2+ imaging and next generation sequencing (RNA-seq) in an effort to understand the determinants of functional plasticity in the skate visual system.
Development of monotypic photoreceptor retinal circuitry
Using a range of methodologies, we are looking at the development of the unique visual system in skate in an effort to understand the fundamental principles of retinal circuit organization. Our goal in this series of projects is to understand how this system has been optimized over evolutionary timescales to perform ecologically relevant functions.